Tissue Analysis Plugin Help
|
Hey all. I have been assigned the task of figuring out how to use image j to count glomeruli in tissue samples of kidney cells. I tried using ITCN but my problem is that the cells I need to count vary in shape, size, and intensity due to the staining. I couldn't find three setting that gave me under 25% error when compared to my hand count.
Here is an example of the image I need to count. Anyone have an idea how I can go about doing this? Thanks 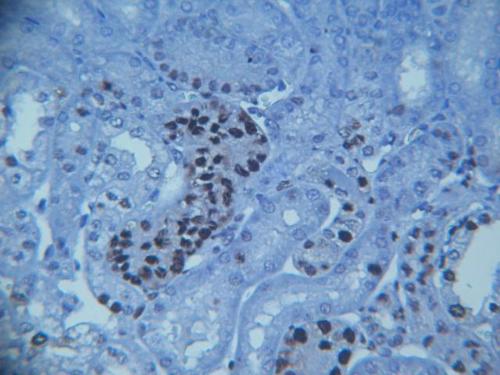
|
Re: Tissue Analysis Plugin Help
|
Hi, I am not familar with this kind of sample and I don't really know
what you try to count, but it looks to me that the illumination across the field of view is not very even. Furthermore, the image appears to lose focus towards the edges, which will all contribute to make it more difficult to automatically detect your objects of interest. You could try to use a background subtraction (Process > Subtract Background) in order to 'flatten' your image, perhaps followed by a bandpass filter (Process > FFT > Bandpass Filter) prior to trying to count your 'glomeruli'. If the objects of interest are the dark stained regions, you could then use either global or local thresholding (Image>Adjust), followed by a particle analysis (Analyze > Analyze Particles) to filter and count your objects. Hope this helps and gives you some ideas, Volko On 15/08/2013 19:39, mnemeh wrote: > Hey all. I have been assigned the task of figuring out how to use image j to > count glomeruli in tissue samples of kidney cells. I tried using ITCN but > my problem is that the cells I need to count vary in shape, size, and > intensity due to the staining. I couldn't find three setting that gave me > under 25% error when compared to my hand count. > > Here is an example of the image I need to count. Anyone have an idea how I > can go about doing this? > > Thanks > > <http://imagej.1557.x6.nabble.com/file/n5004443/Image.jpg> > > > > -- > View this message in context: http://imagej.1557.x6.nabble.com/Tissue-Analysis-Plugin-Help-tp5004443.html > Sent from the ImageJ mailing list archive at Nabble.com. > > -- > ImageJ mailing list: http://imagej.nih.gov/ij/list.html -- ImageJ mailing list: http://imagej.nih.gov/ij/list.html |
Re: Tissue Analysis Plugin Help
|
Hi
Most of that looks like peritubular tissue to me, i.e. I did not see anything that I thought was a glomerulus, what is your Scale and Stain? Kenton On 16 Aug 2013, at 07:33, Volko Straub wrote: > Hi, I am not familar with this kind of sample and I don't really know what you try to count, but it looks to me that the illumination across the field of view is not very even. Furthermore, the image appears to lose focus towards the edges, which will all contribute to make it more difficult to automatically detect your objects of interest. You could try to use a background subtraction (Process > Subtract Background) in order to 'flatten' your image, perhaps followed by a bandpass filter (Process > FFT > Bandpass Filter) prior to trying to count your 'glomeruli'. If the objects of interest are the dark stained regions, you could then use either global or local thresholding (Image>Adjust), followed by a particle analysis (Analyze > Analyze Particles) to filter and count your objects. > Hope this helps and gives you some ideas, > Volko > > > On 15/08/2013 19:39, mnemeh wrote: >> Hey all. I have been assigned the task of figuring out how to use image j to >> count glomeruli in tissue samples of kidney cells. I tried using ITCN but >> my problem is that the cells I need to count vary in shape, size, and >> intensity due to the staining. I couldn't find three setting that gave me >> under 25% error when compared to my hand count. >> >> Here is an example of the image I need to count. Anyone have an idea how I >> can go about doing this? >> >> Thanks >> >> <http://imagej.1557.x6.nabble.com/file/n5004443/Image.jpg> >> >> >> >> -- >> View this message in context: http://imagej.1557.x6.nabble.com/Tissue-Analysis-Plugin-Help-tp5004443.html >> Sent from the ImageJ mailing list archive at Nabble.com. >> >> -- >> ImageJ mailing list: http://imagej.nih.gov/ij/list.html > > -- > ImageJ mailing list: http://imagej.nih.gov/ij/list.html -- ImageJ mailing list: http://imagej.nih.gov/ij/list.html |
Re: Tissue Analysis Plugin Help
|
I concur. No glomeruli in that image.
On Fri, Aug 16, 2013 at 2:41 AM, Kenton Arkill <[hidden email]>wrote: > Hi > Most of that looks like peritubular tissue to me, i.e. I did not see > anything that I thought was a glomerulus, what is your Scale and Stain? > Kenton > > On 16 Aug 2013, at 07:33, Volko Straub wrote: > > > Hi, I am not familar with this kind of sample and I don't really know > what you try to count, but it looks to me that the illumination across the > field of view is not very even. Furthermore, the image appears to lose > focus towards the edges, which will all contribute to make it more > difficult to automatically detect your objects of interest. You could try > to use a background subtraction (Process > Subtract Background) in order to > 'flatten' your image, perhaps followed by a bandpass filter (Process > FFT > > Bandpass Filter) prior to trying to count your 'glomeruli'. If the > objects of interest are the dark stained regions, you could then use either > global or local thresholding (Image>Adjust), followed by a particle > analysis (Analyze > Analyze Particles) to filter and count your objects. > > Hope this helps and gives you some ideas, > > Volko > > > > > > On 15/08/2013 19:39, mnemeh wrote: > >> Hey all. I have been assigned the task of figuring out how to use > image j to > >> count glomeruli in tissue samples of kidney cells. I tried using ITCN > but > >> my problem is that the cells I need to count vary in shape, size, and > >> intensity due to the staining. I couldn't find three setting that gave > me > >> under 25% error when compared to my hand count. > >> > >> Here is an example of the image I need to count. Anyone have an idea > how I > >> can go about doing this? > >> > >> Thanks > >> > >> <http://imagej.1557.x6.nabble.com/file/n5004443/Image.jpg> > >> > >> > >> > >> -- > >> View this message in context: > http://imagej.1557.x6.nabble.com/Tissue-Analysis-Plugin-Help-tp5004443.html > >> Sent from the ImageJ mailing list archive at Nabble.com. > >> > >> -- > >> ImageJ mailing list: http://imagej.nih.gov/ij/list.html > > > > -- > > ImageJ mailing list: http://imagej.nih.gov/ij/list.html > > -- > ImageJ mailing list: http://imagej.nih.gov/ij/list.html > -- ImageJ mailing list: http://imagej.nih.gov/ij/list.html |
|
Thank you all for the help. I will try playing around with those settings
I'm new to this project and since we will be staining/counting glomeruli in the future I assumed that these images that I was given are the same. Sorry about the confusion. I'm not sure what the staining was on these slides (they were given to me to train imagej) but if it is consistent with the project that we will be starting, they used DAB antibody staining. |
Re: Tissue Analysis Plugin Help
|
How many will you be doing? because if it is only several 1000, hand will
probably be quickest (and you'd have to do a hundred or so by hand anyway to test any macro). Gloms are really easy to spot by eye being a specific shape and you could probably do counting by eye very quickly, training a computer I recon would be quite hard. I'd wait until you have some real images regardless and find exactly what the real stain is and how much contrast you have with it. On 17 August 2013 01:46, mnemeh <[hidden email]> wrote: > Thank you all for the help. I will try playing around with those settings > > I'm new to this project and since we will be staining/counting glomeruli in > the future I assumed that these images that I was given are the same. > Sorry > about the confusion. I'm not sure what the staining was on these slides > (they were given to me to train imagej) but if it is consistent with the > project that we will be starting, they used DAB antibody staining. > > > > > > -- > View this message in context: > http://imagej.1557.x6.nabble.com/Tissue-Analysis-Plugin-Help-tp5004443p5004465.html > Sent from the ImageJ mailing list archive at Nabble.com. > > -- > ImageJ mailing list: http://imagej.nih.gov/ij/list.html > -- ImageJ mailing list: http://imagej.nih.gov/ij/list.html |
Re: Tissue Analysis Plugin Help
|
I agree. Automatic is "trendy" - but my advice to collaborators is always:
"If you want me to do computer vision research - this problem is great (we'll have an answer in 2 years; if you want to do "science", here is how to use ImageJ to enhance your images and do the bookkeeping for you while your post-doc provides the eyes." One intermediate strategy I have used lately: build a process to automatically identify (whatever you are counting) and then provide a facility to edit (add, subtract, move, and ClearAll) manually. This strategy provides a method which is always as fast as manual, and sometimes faster. -- Kenneth Sloan [hidden email] On Aug 17, 2013, at 04:04 , Kenton Arkill <[hidden email]> wrote: > How many will you be doing? because if it is only several 1000, hand will > probably be quickest (and you'd have to do a hundred or so by hand anyway > to test any macro). Gloms are really easy to spot by eye being a specific > shape and you could probably do counting by eye very quickly, training a > computer I recon would be quite hard. > > I'd wait until you have some real images regardless and find exactly what > the real stain is and how much contrast you have with it. > > > On 17 August 2013 01:46, mnemeh <[hidden email]> wrote: > >> Thank you all for the help. I will try playing around with those settings >> >> I'm new to this project and since we will be staining/counting glomeruli in >> the future I assumed that these images that I was given are the same. >> Sorry >> about the confusion. I'm not sure what the staining was on these slides >> (they were given to me to train imagej) but if it is consistent with the >> project that we will be starting, they used DAB antibody staining. >> >> >> >> >> >> -- >> View this message in context: >> http://imagej.1557.x6.nabble.com/Tissue-Analysis-Plugin-Help-tp5004443p5004465.html >> Sent from the ImageJ mailing list archive at Nabble.com. >> >> -- >> ImageJ mailing list: http://imagej.nih.gov/ij/list.html >> > > -- > ImageJ mailing list: http://imagej.nih.gov/ij/list.html -- ImageJ mailing list: http://imagej.nih.gov/ij/list.html |
«
Return to ImageJ
|
1 view|%1 views
| Free forum by Nabble | Edit this page |

